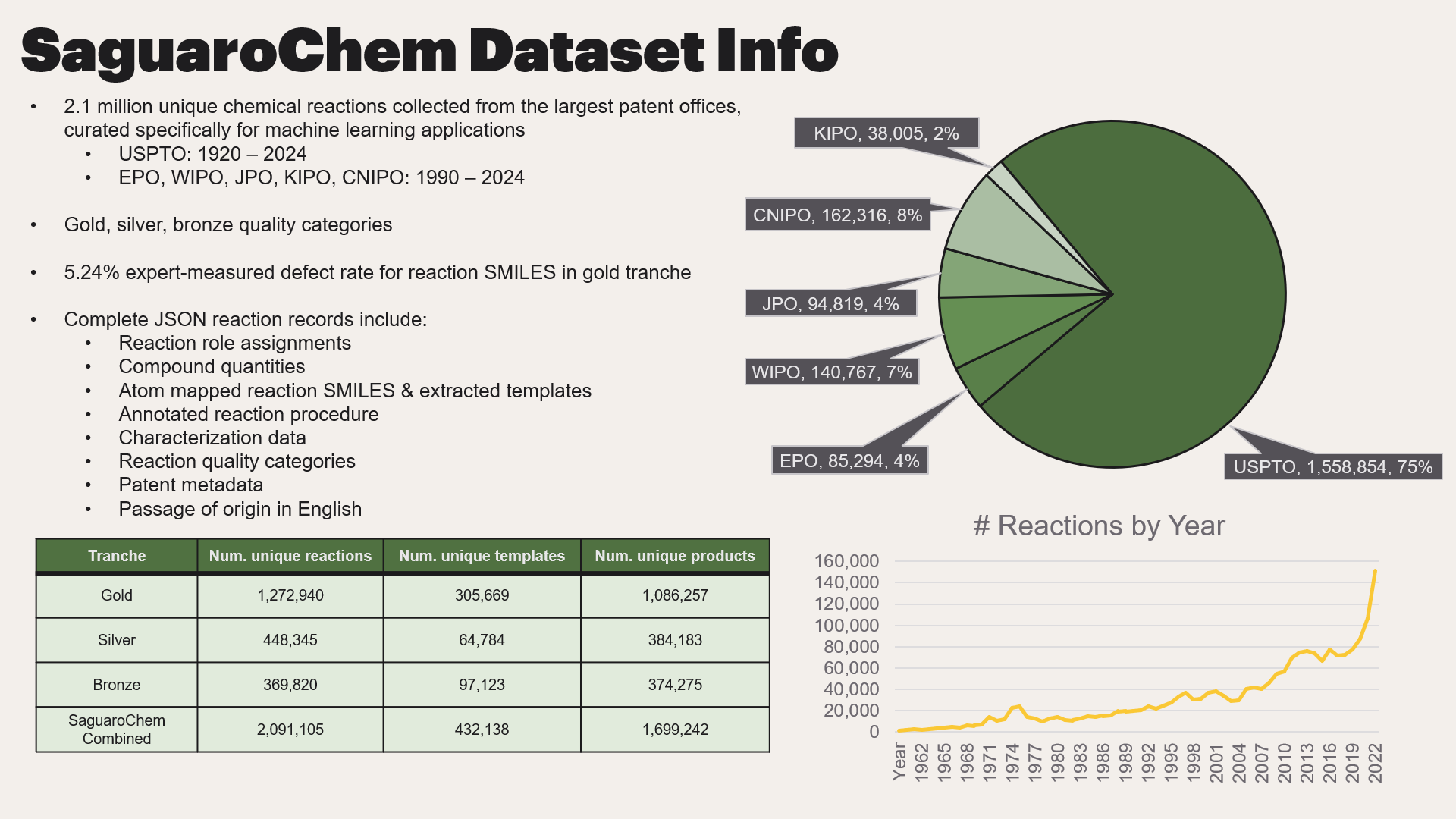
Click here to see a sample reaction record from the SaguaroChem dataset
{'index_num': 1006068,
'passage': '<p> EXAMPLE 134 </p> <p> 2-[3-[2-(2-Aminothiazol-5-yl)ethoxycarbonylamino]-2-oxo-6-phenyl-1,2-dihydr o-1-pyridyl]-N-(3,3,3-trifluoro-1-isopropyl-2-oxopropyl)acetamide. </p> <p> To 2-[3-[2-(2-tert-butoxycarbonylaminothiazol-5-yl)ethoxycarbonylamino]-2-oxo -6-phenyl-1,2-dihydro-1-pyridyl]-N-(3,3,3-trifluoro-1-isopropyl-2-oxopropyl )acetamide (0.9 g) in methylene chloride (5 mL) was added trifluoroacetic acid (1 mL), and the resulting solution was allowed to stir for 3 h. The solvents were evaporated and the residue chromatographed, eluting with methanol:methylene chloride (5:95), to give the title compound (0.46 g); MS: m/z=566(M+1). </p>',
'metadata': {'wku': '055211798',
'url': 'https://patents.google.com/patent/US5521179',
'title': 'Heterocyclic amides',
'asignee': 'AstraZeneca UK Ltd',
'authors': ['Peter R. Bernstein',
'Andrew Shaw',
'Royston M. Thomas',
'Peter Warner',
'Donald J. Wolanin'],
'filings_other_offices': ['GB929217363A',
'GB929205392A',
'GB919108357A',
'GB929214448A',
'GB929208380A',
'GB929217362A',
'GB929208379A',
'US08/045,009',
'GB929217364A',
'GB919108358A'],
'language': 'english',
'year_assigned': '1991-04-18',
'year_granted': '1996-05-28',
'publication_type': 'Patent',
'publication_name': 'USPTO'},
'tranche': 'gold',
'reaction_roles': [{'reaction-roles': [{'reactants': [{'name': '2-[3-[2-(2-tert-butoxycarbonylaminothiazol-5-yl)ethoxycarbonylamino]-2-oxo -6-phenyl-1,2-dihydro-1-pyridyl]-N-(3,3,3-trifluoro-1-isopropyl-2-oxopropyl )acetamide',
'alt-name': '',
'mass': {'value': '0.9', 'unit': 'gram'},
'volume': {'value': '', 'unit': ''},
'molar quantity': {'value': '', 'unit': ''},
'SMILES': 'CC(C)C(NC(=O)Cn1c(-c2ccccc2)ccc(NC(=O)OCCc2cnc(NC(=O)OC(C)(C)C)s2)c1=O)C(=O)C(F)(F)F'},
{'name': 'trifluoroacetic acid',
'alt-name': '',
'mass': {'value': '', 'unit': ''},
'volume': {'value': '1', 'unit': 'milliliter'},
'molar quantity': {'value': '', 'unit': ''},
'SMILES': 'O=C(O)C(F)(F)F'}],
'solvents': [{'name': 'methylene chloride',
'alt-name': '',
'mass': {'value': '', 'unit': ''},
'volume': {'value': '5', 'unit': 'milliliter'},
'molar quantity': {'value': '', 'unit': ''},
'SMILES': 'ClCCl'}],
'catalysts': [],
'intermediates': [],
'workup_reagents': [],
'byproducts': [],
'products': [{'name': '2-[3-[2-(2-Aminothiazol-5-yl)ethoxycarbonylamino]-2-oxo-6-phenyl-1,2-dihydr o-1-pyridyl]-N-(3,3,3-trifluoro-1-isopropyl-2-oxopropyl)acetamide',
'alt-name': 'EXAMPLE 134',
'mass': {'value': '0.46', 'unit': 'gram'},
'volume': {'value': '', 'unit': ''},
'molar quantity': {'value': '', 'unit': ''},
'yield': '',
'SMILES': 'CC(C)C(NC(=O)Cn1c(-c2ccccc2)ccc(NC(=O)OCCc2cnc(N)s2)c1=O)C(=O)C(F)(F)F'}]}],
'reactants_list': ['CC(C)C(NC(=O)Cn1c(-c2ccccc2)ccc(NC(=O)OCCc2cnc(NC(=O)OC(C)(C)C)s2)c1=O)C(=O)C(F)(F)F',
'O=C(O)C(F)(F)F'],
'solvents_list': ['ClCCl'],
'catalysts_list': [],
'byproducts_list': [],
'products_list': ['CC(C)C(NC(=O)Cn1c(-c2ccccc2)ccc(NC(=O)OCCc2cnc(N)s2)c1=O)C(=O)C(F)(F)F'],
'intermediates_list': [],
'workup_reagents_list': [],
'rxn_smiles': 'CC(C)C(NC(=O)Cn1c(-c2ccccc2)ccc(NC(=O)OCCc2cnc(NC(=O)OC(C)(C)C)s2)c1=O)C(=O)C(F)(F)F.O=C(O)C(F)(F)F.ClCCl>>CC(C)C(NC(=O)Cn1c(-c2ccccc2)ccc(NC(=O)OCCc2cnc(N)s2)c1=O)C(=O)C(F)(F)F',
'graphformer_mapped_smiles': '[CH3:35][C:34]([CH3:36])([CH3:37])[O:33][C:31]([NH:30][c:29]1[n:28][cH:27][c:26]([CH2:25][CH2:24][O:23][C:21]([NH:20][c:19]2[cH:18][cH:17][c:10]([n:9]([CH2:8][C:6](=[O:7])[NH:5][CH:4]([CH:2]([CH3:1])[CH3:3])[C:41](=[O:42])[C:43]([F:44])([F:45])[F:46])[c:39]2=[O:40])-[c:11]2[cH:16][cH:15][cH:14][cH:13][cH:12]2)=[O:22])[s:38]1)=[O:32].[Cl:56][CH2:55][Cl:54].[F:51][C:50]([F:52])([F:53])[C:48]([OH:49])=[O:47]>>[F:44][C:43]([F:45])([F:46])[C:41](=[O:42])[CH:4]([CH:2]([CH3:1])[CH3:3])[NH:5][C:6]([CH2:8][n:9]1[c:10]([cH:17][cH:18][c:19]([c:39]1=[O:40])[NH:20][C:21]([O:23][CH2:24][CH2:25][c:26]1[cH:27][n:28][c:29]([NH2:30])[s:38]1)=[O:22])-[c:11]1[cH:16][cH:15][cH:14][cH:13][cH:12]1)=[O:7]',
'rxn_mapper_mapped_smiles': '[CH3:1][CH:2]([CH3:3])[CH:4]([NH:5][C:6](=[O:7])[CH2:8][n:9]1[c:10](-[c:11]2[cH:12][cH:13][cH:14][cH:15][cH:16]2)[cH:17][cH:18][c:19]([NH:20][C:21](=[O:22])[O:23][CH2:24][CH2:25][c:26]2[cH:27][n:28][c:29]([NH:30]C(=O)OC(C)(C)C)[s:31]2)[c:32]1=[O:33])[C:34](=[O:35])[C:36]([F:37])([F:38])[F:39].O=C(O)C(F)(F)F.ClCCl>>[CH3:1][CH:2]([CH3:3])[CH:4]([NH:5][C:6](=[O:7])[CH2:8][n:9]1[c:10](-[c:11]2[cH:12][cH:13][cH:14][cH:15][cH:16]2)[cH:17][cH:18][c:19]([NH:20][C:21](=[O:22])[O:23][CH2:24][CH2:25][c:26]2[cH:27][n:28][c:29]([NH2:30])[s:31]2)[c:32]1=[O:33])[C:34](=[O:35])[C:36]([F:37])([F:38])[F:39]',
'local_mapper_mapped_smiles': '[CH3:1][CH:2]([CH3:3])[CH:4]([NH:5][C:6](=[O:7])[CH2:8][n:9]1[c:10](-[c:11]2[cH:12][cH:13][cH:14][cH:15][cH:16]2)[cH:17][cH:18][c:19]([NH:20][C:21](=[O:22])[O:23][CH2:24][CH2:25][c:26]2[cH:27][n:28][c:29]([NH:30]C(=O)OC(C)(C)C)[s:31]2)[c:32]1=[O:33])[C:34](=[O:35])[C:36]([F:37])([F:38])[F:39].O=C(O)C(F)(F)F.ClCCl>>[CH3:1][CH:2]([CH3:3])[CH:4]([NH:5][C:6](=[O:7])[CH2:8][n:9]1[c:10](-[c:11]2[cH:12][cH:13][cH:14][cH:15][cH:16]2)[cH:17][cH:18][c:19]([NH:20][C:21](=[O:22])[O:23][CH2:24][CH2:25][c:26]2[cH:27][n:28][c:29]([NH2:30])[s:31]2)[c:32]1=[O:33])[C:34](=[O:35])[C:36]([F:37])([F:38])[F:39]',
'rxn_mapper_template': '[#16;a:3]:[c:2](-[NH2;D1;+0:1]):[#7;a:4]>>C-C(-C)(-C)-O-C(=O)-[NH;D2;+0:1]-[c:2](:[#16;a:3]):[#7;a:4]',
'rxn_mapper_reactants': 'CC(C)C(NC(=O)Cn1c(-c2ccccc2)ccc(NC(=O)OCCc2cnc(NC(=O)OC(C)(C)C)s2)c1=O)C(=O)C(F)(F)F',
'rxn_mapper_spectators': 'O=C(O)C(F)(F)F.ClCCl',
'local_mapper_template': '[#16;a:3]:[c:2](-[NH2;D1;+0:1]):[#7;a:4]>>C-C(-C)(-C)-O-C(=O)-[NH;D2;+0:1]-[c:2](:[#16;a:3]):[#7;a:4]',
'local_mapper_reactants': 'CC(C)C(NC(=O)Cn1c(-c2ccccc2)ccc(NC(=O)OCCc2cnc(NC(=O)OC(C)(C)C)s2)c1=O)C(=O)C(F)(F)F',
'local_mapper_spectators': 'O=C(O)C(F)(F)F.ClCCl',
'graphformer_mapper_template': '[#16;a:3]:[c:2](-[NH2;D1;+0:1]):[#7;a:4]>>C-C(-C)(-C)-O-C(=O)-[NH;D2;+0:1]-[c:2](:[#16;a:3]):[#7;a:4]',
'graphformer_mapper_reactants': 'CC(C)C(NC(=O)Cn1c(-c2ccccc2)ccc(NC(=O)OCCc2cnc(NC(=O)OC(C)(C)C)s2)c1=O)C(=O)C(F)(F)F',
'graphformer_mapper_spectators': 'ClCCl.O=C(O)C(F)(F)F',
'consensus_template': '[#16;a:3]:[c:2](-[NH2;D1;+0:1]):[#7;a:4]>>C-C(-C)(-C)-O-C(=O)-[NH;D2;+0:1]-[c:2](:[#16;a:3]):[#7;a:4]'}],
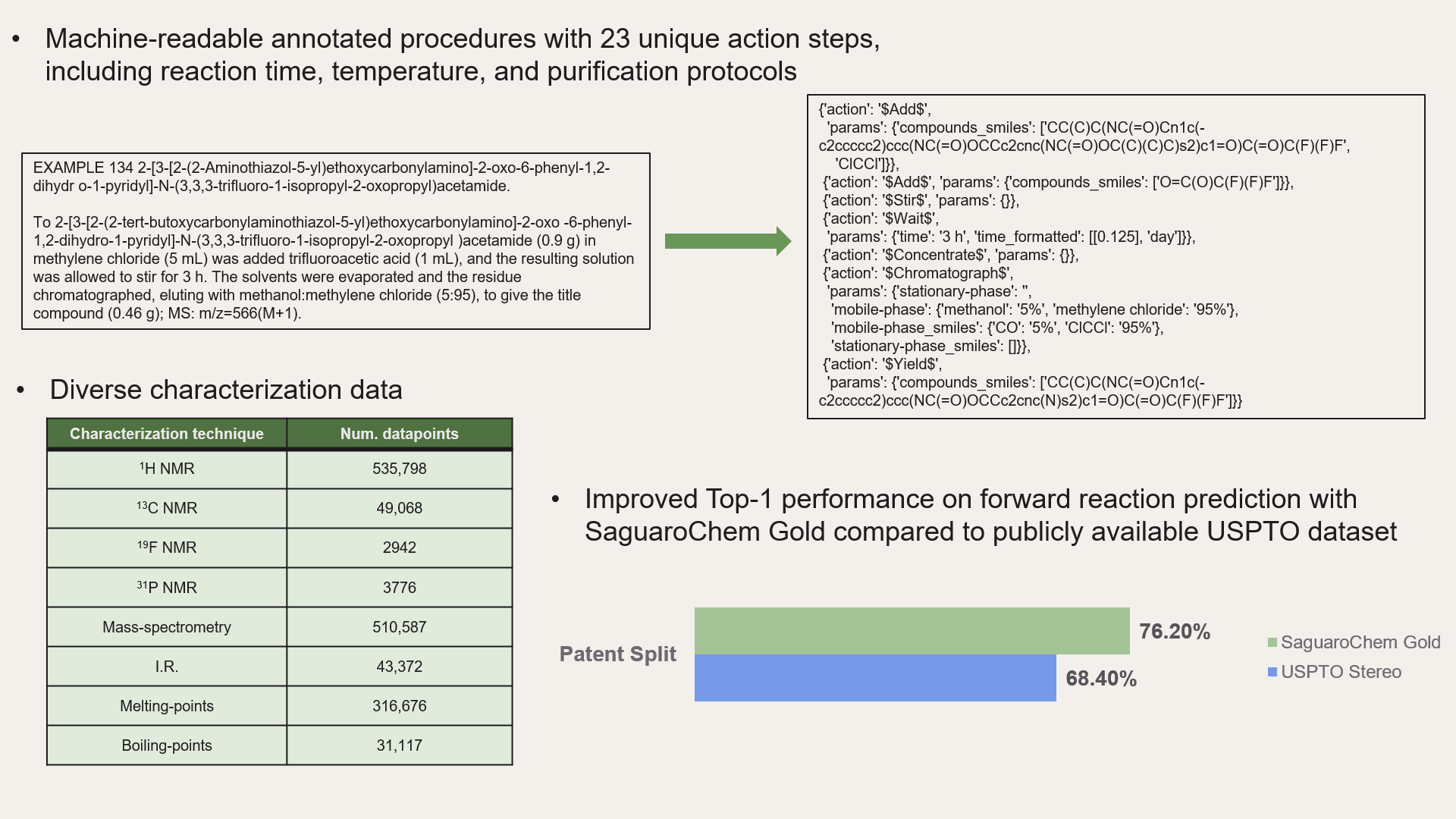
'procedure': [{'action': '$Add$',
'params': {'compounds_smiles': ['CC(C)C(NC(=O)Cn1c(-c2ccccc2)ccc(NC(=O)OCCc2cnc(NC(=O)OC(C)(C)C)s2)c1=O)C(=O)C(F)(F)F',
'ClCCl']}},
{'action': '$Add$', 'params': {'compounds_smiles': ['O=C(O)C(F)(F)F']}},
{'action': '$Stir$', 'params': {}},
{'action': '$Wait$',
'params': {'time': '3 h', 'time_formatted': [[0.125], 'day']}},
{'action': '$Concentrate$', 'params': {}},
{'action': '$Chromatograph$',
'params': {'stationary_phase': '',
'mobile_phase': {'methanol': '5%', 'methylene chloride': '95%'},
'mobile_phase_smiles': {'CO': '5%', 'ClCCl': '95%'},
'stationary_phase_smiles': []}},
{'action': '$Yield$',
'params': {'compounds_smiles': ['CC(C)C(NC(=O)Cn1c(-c2ccccc2)ccc(NC(=O)OCCc2cnc(N)s2)c1=O)C(=O)C(F)(F)F']}}],
'characterization_data': {'MS': 'm/z=566(M+1)'}}See what’s possible with the SaguaroChem dataset – check out our demos for forward reaction prediction, procedure retrosynthesis prediction, purification protocol prediction, and more
Get access to the SaguaroChem dataset
Non-derivative License
Train models for internal use with SaguaroChem
Academic License
Conduct academic research with SaguaroChem
Contact us for a detailed quote
Still not sure? Ask about obtaining 500 reaction records for evaluation
